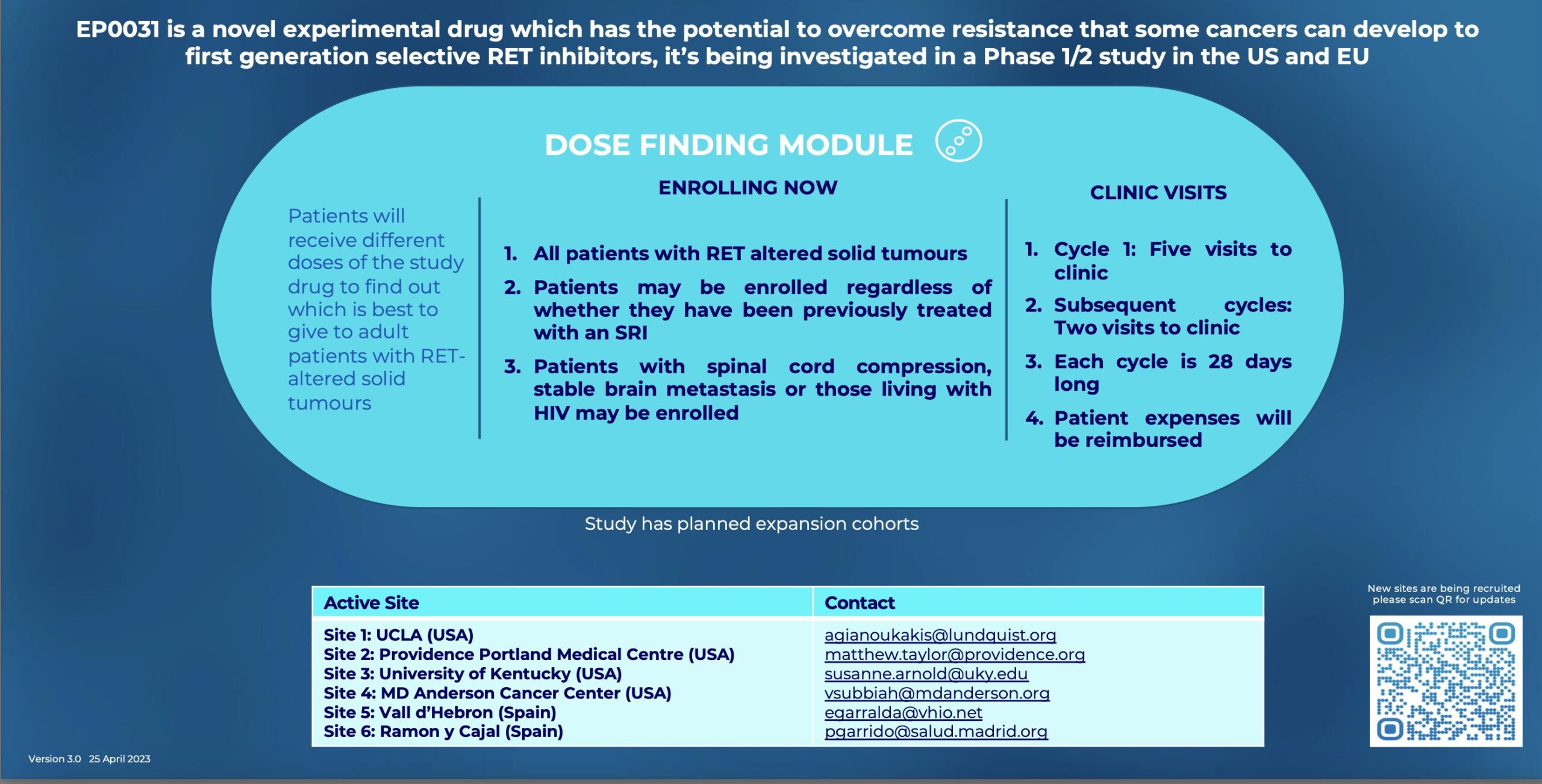
Data of the Phase 1 Study of KL590586 (EP0031/A400), presented at ASCO, reported preliminary efficacy and safety for a total of 109 patients. EP0031/A400 is a potent next generation specific RET inhibitor with broad activity against common RET fusions and mutations, including solvent front resistance mutations. Therefore, EP0031/A400 may have the potential to overcome resistance to first generation RET inhibitors.
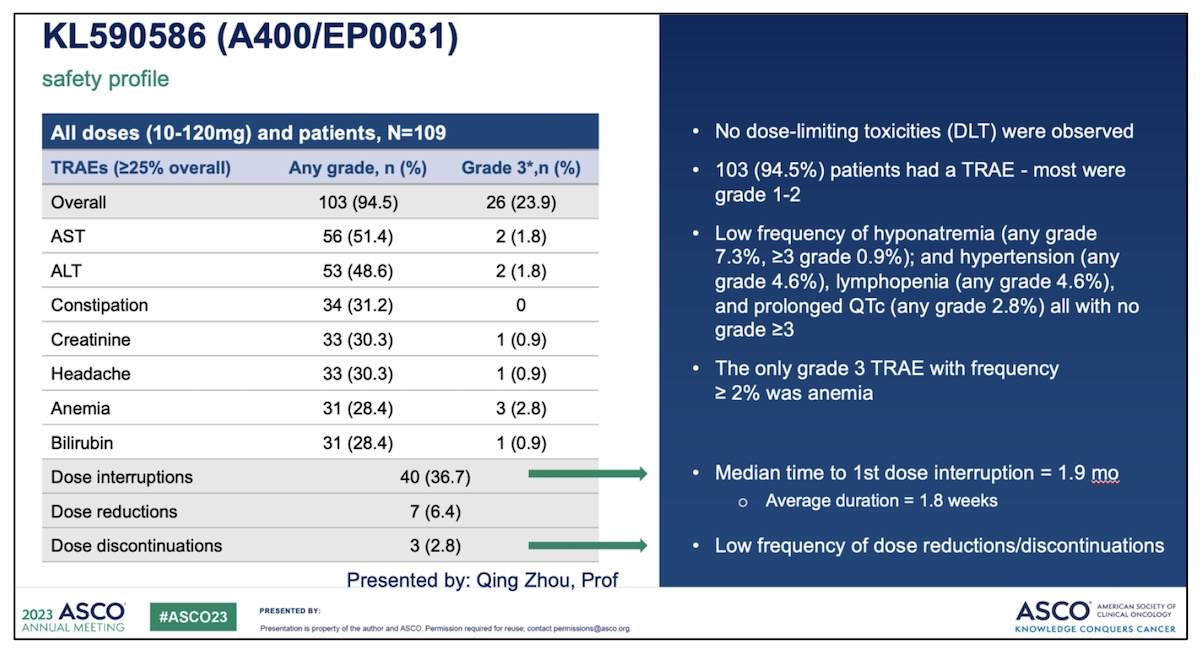
Safety: A400/EP0031 was generally well tolerated with most treatment-related adverse events grade 1-2.
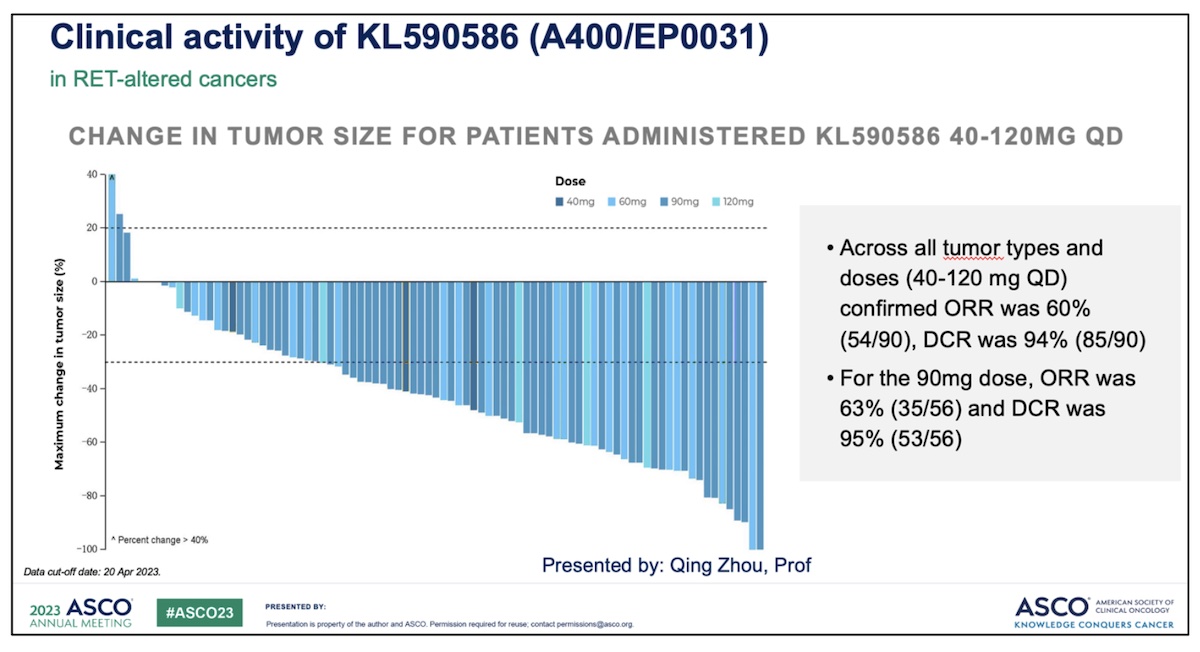
Efficacy: In the overall RET-altered tumor population, patients who received A400/EP0031 at doses between 40 and 120mg once a day had an objective response rate of 60% with a disease control rate of 90%.
Two specific treatment cohorts were highlighted:
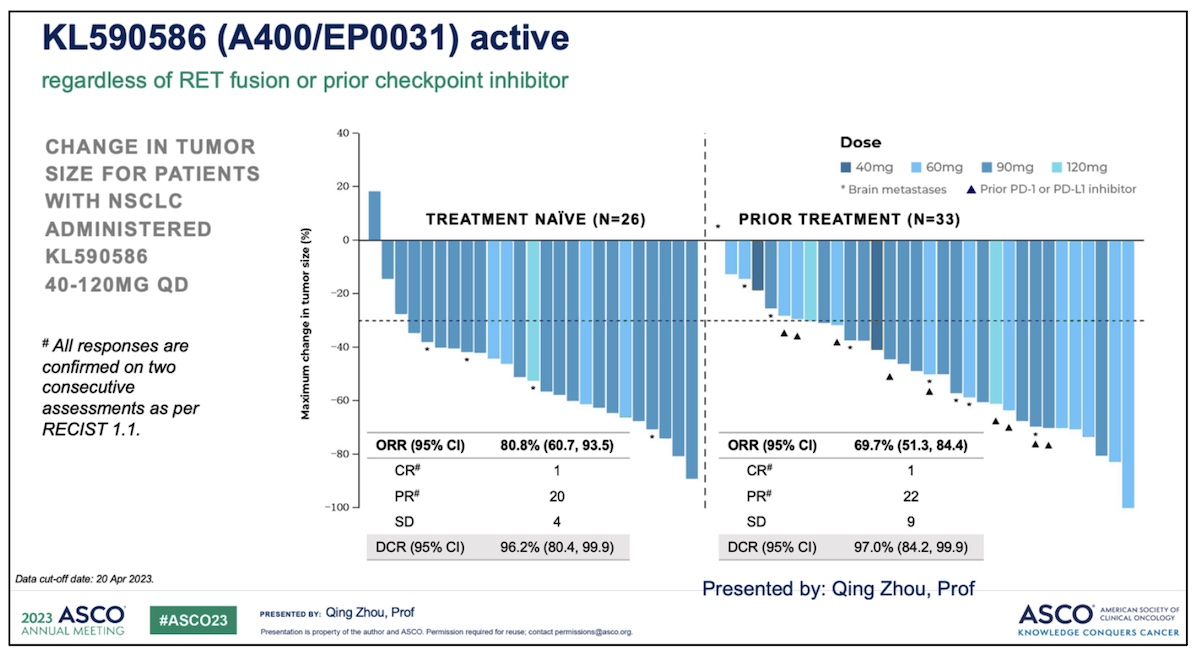
- Patients, with previously untreated RET-fusion positive advanced NSCLC with an objective response rate of 80.8% (21/26 patients).
- Patients with RET-fusion positive NSCLC who had received prior systemic treatment, including chemo- immunotherapy, with an objective response rate of 69.7% (23/33 patients). Disease control rates of >96% were reported for each cohort.
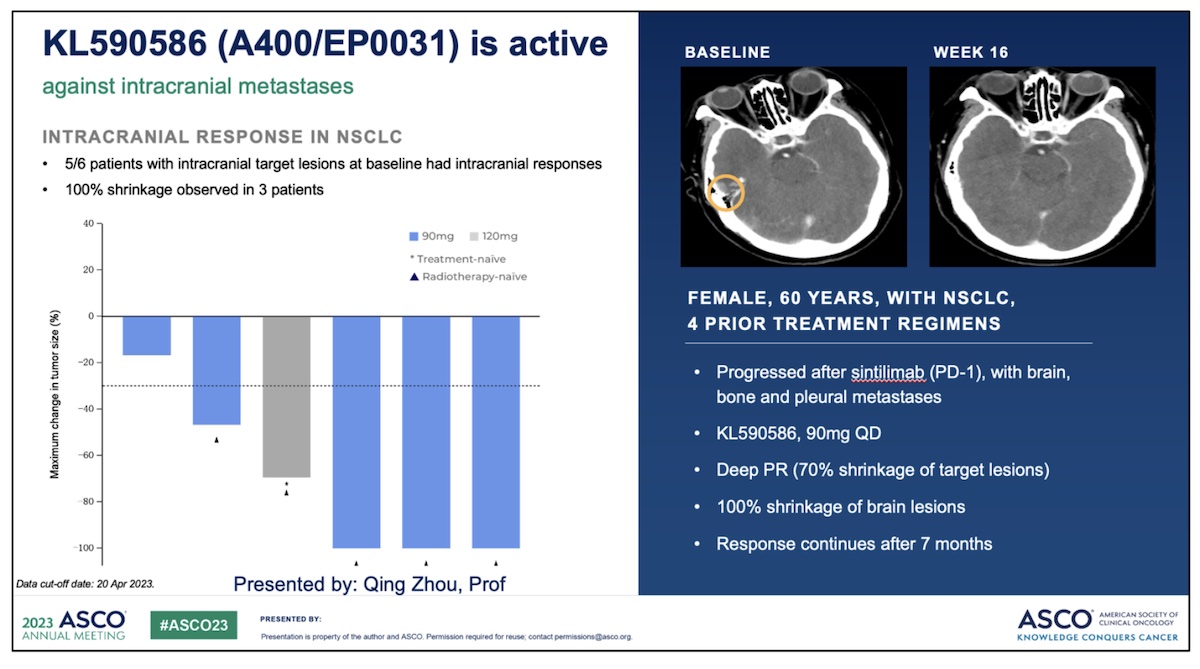
Importantly, evidence of clinical activity was also reported in cohorts of patients with brain metastases as well as patients that had received prior 1st generation SRI.
EP0031/A400 is the subject of a global, modular Phase 1/2 trial to evaluate safety, tolerability and efficacy in patients with advanced RET-altered tumors. The study is open in multiple sites across the US (NCT05443126).