Cancer drug resistance means that a tumor no longer responds to anticancer drugs that were once effective. This can be a significant challenge, as it may limit treatment options and affect the patient’s prognosis.
Understanding Drug Resistance in Cancer Therapy
Targeted cancer therapies can be very effective in eliminating cancer cells, but unfortunately some tumor cells may escape the effect of the treatment and survive. The surviving cells are able to proliferate and drive tumor growth.
RET targeted therapies have shown promise in treating RET cancer. These medications specifically target the abnormal RET proteins, slowing cancer progression and improving patient outcomes. However, despite initial success, some patients develop resistance to these cancer treatments over time.
Signs of Resistance to Cancer Treatment
Signs that a treatment may be losing its effectiveness include the return or worsening of symptoms, such as increased coughing, difficulty breathing, or new pain. Regular monitoring through scans and tests is vital to detect resistance early and adjust the treatment plan accordingly.
Resistance to Selective RET Kinase Inhibition
Targeted cancer therapies, including the FDA-approved selective RET inhibitors that specifically target or inhibit the RET molecule – selpercatinib (Retevmo) and pralsetinib (Gavreto) – have transformed the treatment of RET cancer patients. These compounds specifically target the RET protein and have shown great clinical benefit in RET fusion positive NSCLC patients, with high response rates in pretreated patients and in treatment naïve patients. They also showed a high intracranial efficacy in RET patients with brain metastases (1-3).
Previous to the development of these agents and their approval in 2020, the therapeutic options for RET fusion-positive NSCLC patients were scarce, with the standard treatment being chemotherapy drugs or immunotherapy. Some multi-kinase inhibitors that target RET were investigated for the treatment of RET cancers but the effectiveness was low and they showed some toxicity with a high number of patients requiring discontinuation of the treatment (4).
Like what happens with other selective inhibitors and despite the clinical benefit of selective RET inhibitors, within 1-3 years many RET lung cancer patients become resistant to these targeted therapies and the cancer progresses again (5). Unfortunately, there is no approved therapy to treat patients who are resistant to RET inhibitors.
RET patients can develop resistance by two main underlying mechanisms, as described here:
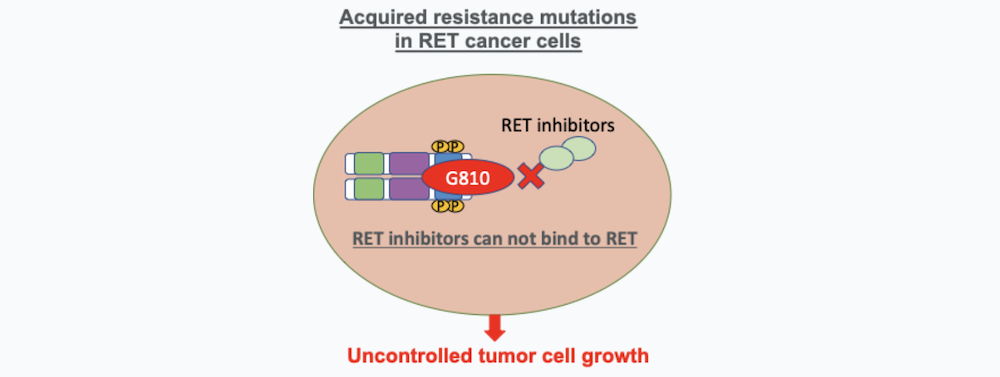
1. Acquired Resistance Mutations in RET Cancer Cells
RET cancer treatment resistance can happen by newly acquired mutations in the RET molecule that are developed after the treatment with selective RET targeted therapy and produce an alteration on the RET molecule that allow the cancer cell to escape the effects of targeted drugs. These mutations produce a modification of the RET molecule that avoids the therapeutic drug to bind effectively. Some of the resistant mutations described include the RET molecule solvent front mutations (RET G810 mutations) or hinge mutations. Acquired mutations confer RET acquired drug resistance, and they are found in some patients treated with selpercatinib or pralsetinib (6, 7).
How to overcome acquired cancer drug resistance?
Investigators are now developing and testing new second-generation RET inhibitors that are able to inhibit these RET resistant mutations in cancer cells, overcoming resistance. Examples of these new targeted cancer therapies and clinical trials are:
RET inhibitor EP0031
EP0031 is a next generation specific RET inhibitor developed by Ellipses Pharma with activity against common RET fusions and mutations, including solvent front mutations that confer drug resistance of cancer cells. The Phase 1/2 trial evaluating safety, tolerability and efficacy in patients with advanced RET-altered tumors is ongoing (NCT05443126).
RET Inhibitor vepafestinib
The RET Inhibitor vepafestinib (TAS0953/HM06) developed by Helsinn Healthcare is a RET-specific inhibitor that is effective against RET solvent front (G810) mutations. A recent study led by Dr. Romel Somwar (MSKCC) showed great efficacy of vepafestinib in RET preclinical models, including RET cancer cell and mouse models bearing RET drug resistance mutations and superior pharmacokinetic properties in the brain (8). The brain is a common site of relapse for patients with RET NSCLC treated with targeted therapies. The phase I clinical trial is ongoing (NCT04683250).
RET Inhibitor APS03118
The RET Inhibitor APS03118 developed by Applied Pharmaceutical Science APS03118 is a novel next-generation RET inhibitor that targets a range of RET fusions and mutations in cancer cells including drug resistance mutations. The Phase 1 clinical trial in patients with advanced RET-altered tumors is ongoing (NCT05653869) (Only China locations).
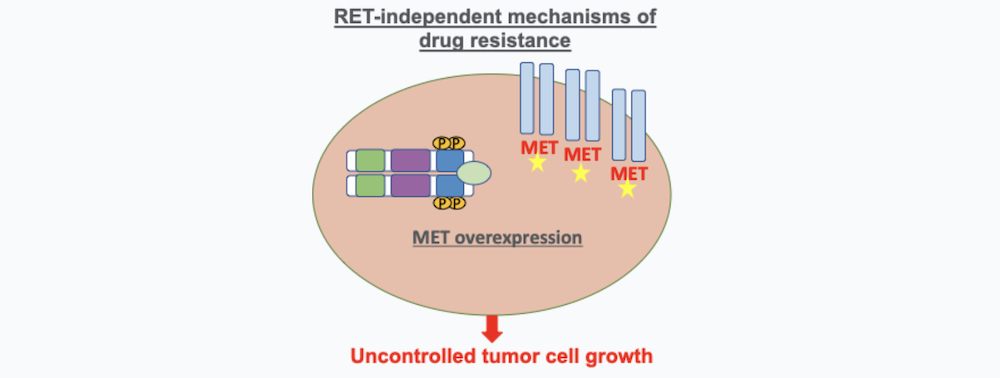
2. RET-Independent Mechanisms of Drug Resistance
These mechanisms of resistance are caused by an over-expression of pathways of cancer cells that are independent of RET that can also cause cancer cells to proliferation. One of the main pathways that has been characterized to produce resistance to RET inhibitors is MET amplification (9). A study led by Dr. Jessica Lin demonstrated that the majority of RET drug resistance is mediated by these RET-independent resistance mechanisms highlighting the urgent need of finding new therapies or more effective combination therapy for RET inhibitor resistance that is mediated by activation of alternative pathways (9).
How to overcome RET-independent cancer drug resistance?
Investigators are now testing combinations of drugs that target the over expressed molecules like MET along with RET inhibitors in RET cancer cells with the aim to overcome drug resistance.
Here is an example of a clinical trial that is testing combinations for RET cancer:
Amivantamab in combination with RET inhibitors
A Phase 1 / 2 study testing amivantamab, a bispecific antibody that targets epidermal growth factor receptor (EGFR) and MET in combination with RET inhibitors for patients who progressed on RET therapies (NCT05845671). In many cases, patients who become resistant to targeted therapies including RET inhibitors present increased activation of EGFR or MET as a bypass signaling mechanism that allows these cancer cells to circumvent the selective pressure from the therapy. The new clinical trial lead by Dr. Tejas Patil from University of Colorado, study the effects of amivantamab, a bispecific antibody that binds to the extracellular domains of EGFR and MET in patients who progressed on TKI therapies including RET therapies.
Future Directions in RET Drug Resistance Research
It is critical to develop new therapeutic strategies that prevent resistance or offer effective treatment options for RET patients who become resistant to selpercatinib and pralsetinib. Our funded investigators are actively investigating new therapies for RET-inhibitor resistant disease.
Progress of our Funded Investigators Tackling RET Drug Resistance
Investigators performed a large characterization of RET tumor cells and found that certain RET fusions are more sensitive to specific RET inhibitors. This is clinically relevant and could potentially determine optimal cancer therapies for RET inhibitor resistant patients bearing specific mutations. Also, by using an innovative approach called Lentimutate they were able to successfully predict therapeutic resistance of a panel of RET cancer cell lines with different RET fusions. Additionally, the investigators have generated in the lab valuable RET cancer cells that are resistant to RET inhibitors. The team is studying new therapeutic targets in these RET cancer cells and how they can be targeted by using FDA-approved cancer therapies or inhibitors that currently being tested on clinical trials. In the case that there are not available therapies or inhibitors, the investigators will design new agents to target these molecules for RET cancer cells and tumors. View the 2023 RET cancer research update.
Don’t Give Up Hope
Understanding and managing treatment resistance is crucial for those with RET cancer. By staying informed about current treatments and emerging options, patients can work with their healthcare team to navigate this challenging aspect of the cancer journey.
If you or a loved one is facing RET cancer, staying informed and proactive in your treatment is essential. Reach out to your healthcare providers for support and explore available resources to help manage your lung cancer journey.
Match to a RET clinical trial
View latest RET treatment options
References
- Drilon A. et al. Efficacy of Selpercatinib in RET Fusion-Positive NSCLC. N Engl J Med. 27;383(9):813-824 (2020).
- Zhou C, Solomon B, Loong HH, et al. First-Line Selpercatinib or Chemotherapy and Pembrolizumab in RET Fusion-Positive NSCLC. N Engl J Med. 2023;389(20):1839-1850. doi:10.1056/NEJMoa2309457
- Gainor J.F. et al. Pralsetinib for RET fusion-positive NSCLC (ARROW): a multi-cohort, open-label, phase 1/2 study. Lancet Oncol. 22(7):959-969 (2021).
- Drilon A, Rekhtman N, Arcila M, et al. Cabozantinib in patients with advanced RET-rearranged non-small-cell lung cancer: an open-label, single-centre, phase 2, single-arm trial. Lancet Oncol. 2016;17(12):1653-1660. doi:10.1016/S1470-2045(16)30562-9
- Lin JJ, Gainor JF. An early look at selective RET inhibitor resistance: new challenges and opportunities. Br J Cancer. 2021;124(11):1757-1758. doi:10.1038/s41416-021-01344-7
- Solomon BJ, Tan L, Lin JJ, et al. RET Solvent Front Mutations Mediate Acquired Resistance to Selective RET Inhibition in RET-Driven Malignancies. J Thorac Oncol. 2020;15(4):541-549. doi:10.1016/j.jtho.2020.01.006
- Subbiah V, Shen T, Terzyan SS, et al. Structural basis of acquired resistance to selpercatinib and pralsetinib mediated by non-gatekeeper RET mutations. Ann Oncol. 2021;32(2):261-268. doi:10.1016/j.annonc.2020.10.599
- Miyazaki I, Odintsov I, Ishida K, et al. Vepafestinib is a pharmacologically advanced RET-selective inhibitor with high CNS penetration and inhibitory activity against RET solvent front mutations [published correction appears in Nat Cancer. 2023 Oct;4(10):1526]. Nat Cancer. 2023;4(9):1345-1361. doi:10.1038/s43018-023-00630-y
- Lin JJ, Liu SV, McCoach CE, et al. Mechanisms of resistance to selective RET tyrosine kinase inhibitors in RET fusion-positive non-small-cell lung cancer. Ann Oncol. 2020;31(12):1725-1733. doi:10.1016/j.annonc.2020.09.015
Your donation matters. Please make a gift today to support lung cancer research and save lives.